The Omicron Medical Department
Omicron’s Medical Division consolidates more than 30 years of business experience in the field of developing and producing industrial lasers and systems for LifeSciences. For now over 10 years, Omicron has been a certified manufacturer of medical laser systems e.g. for PDT (photodynamic therapy) applications. The Omicron Medical business unit combines both industrial and medical background to provide excellent technologies and services. Key benefits for our customers are the company’s knowledge and experience in laser applications and the strong support in attending customers during their projects and developments. Customization as well as OEM business together with reliable quality result in the major competences Omicron is known for.
Our productsOmicron Medical Products


What you can expect:
ISO Certified production
Omicron designs and manufactures medical lasers according to ISO 13485:2016 with technical approval according to the EN (IEC) 60601-1 (Edition 3.1) standard. Further the design complies to FDA/CDRH 21 CFR 1040.10 and Japanese PMDA standards.
High-competence consultation
With more than 30 years of experience in industrial laser technology and over 10 years as a manufacturer for medical laser systems Omicron provides comprehensive advice for various challenges in the market.
Customized solutions
Omicron supports any kind of customized solutions. Developing new products or adapting existing designs to customer´s requirements is one of the core capabilities. From prototype to serial production, Omicron is your competent partner.
A reliable partner
Being a reliable partner in long term business relations or supporting start-ups, Omicron helps and consults during standard processes and challenging new upcoming projects.
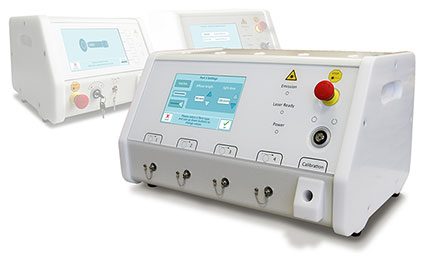
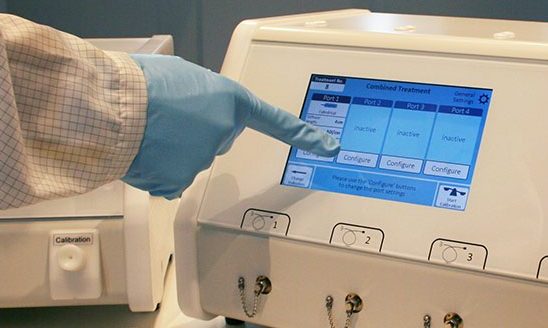
PDT Laser Systems
For activation of Photosensitizers intended for the Treatment of Cancer
Omicron offers laser systems for PDT applications in combination with common or new photosensitizers.
The systems are equipped with all required functionalities for usability and safety. The perfect match for common or new photosensitizers.CE Certified Designed and manufactured according to ISO13485:2016 Technical approval according to the EN (IEC) 60601-1 (Edition 3.1) Design complies to US-FDA0/CDRH 21 CFR 1040.10 & Japanese MHLW medical device standards and
QMS ISO13485 approved by PMDA
Company Philosophy
The benchmark for the quality of our products and services is determined by the customer. Their judgement is decisive. Through our knowledge and know-how of medical devices, we have a moral obligation to develop and manufacture them for the benefit of patients.
OUR WISHES
– to deliver customized solutions for individual requirements – to provide customized support – to always be one step ahead of competitors – to bring a new development to the market twice a year – to be a technology leader – to develop „unique“ products – to offer exceptional after sales support – to provide individual demonstration systems
WE ACHIEVE THIS BY
– the quality of our products and services – the ability to work in a team – a friendly approach to our customers, suppliers and colleagues – efforts to constantly improve ourselves and our processes within the company
FOR THE PURPOSE OF
– being perceived by our customers as a friendly, customer-oriented company, which works in accordance with their interests and delivers high-quality products – being regarded as a reliable partner for customers and suppliers at home and abroad – building long-lasting and stable relationships through high customer satisfaction

Never stop exploring
Omicron introduces the new inhouse TechCamp. As an institution for seminars, workshops and product presentations the TechCamp comes up with high tech equipment like advanced meeting rooms as well as movable and flexible glass walls for larger conferences. Customers and partners can explore product service, a complete microscopy room and an entire virtual reality operating theatre.
Get in touch
One world
Omicron Medical Japan is a branch establishment representing Omicron Medical products exclusively in Japan. The operating base is located in Tokyo. OMJ is officially licensed by the Japanese authorities to service and repair all medical products.
Get in touchLatest News
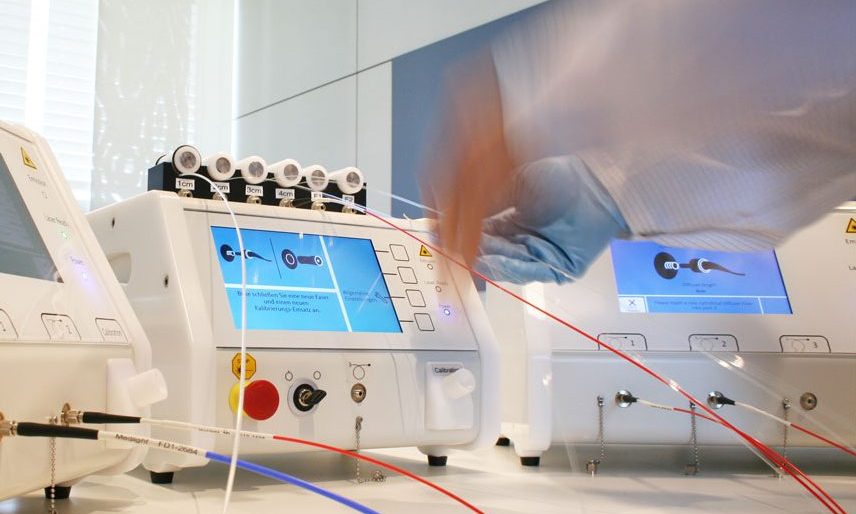
Caligauge: “Stand-alone fibre calibration port”
Key features:
– Measurement of optical output power for frontal and cylindrical diffusers
– Measurement of fibre transmission with pass/fail indication
– Exact measurement of fluence rate at application/treatment spot